Boiling Point Of Water / Distillation And Boiling Points Fsc 432 Petroleum Refining : For instance, at sea level, the air pressure is 1 atm.
Boiling Point Of Water / Distillation And Boiling Points Fsc 432 Petroleum Refining : For instance, at sea level, the air pressure is 1 atm.. Calculate the boiling point of a solution of 10 grams of sodium chloride in 200 grams of water. Reference tables contain values of the boiling point of water at different pressures (in different unit of measure). Assume the following conditions for water. The quantity of heat required to completely vaporise a unit mass of a liquid gas at its boiling point is called latent heat of vaporisation of the liquid. When water is heated slowly enough, air bubbles are noticed forming on the sides and bottom of the pan.
Under this condition, addition of heat results in the transformation of the liquid into its vapor without raising water at its boiling point. It is represented by the symbol l. The boiling point for water at sea level and under standard conditions is 100 degrees celsius (212f). By how much depends on how much salt there is. Putting the water in a pressurized container raises the boiling point, and putting it in a vacuum lowers the boiling point.
Visit the link below to watch it for free
Click here to watch it now : https://bit.ly/2NpXrtG
The temperature at which substance change from liquid to the gaseous state is known as the boiling point. It can boil at 0 degrees if you really want it to, and it isn't sheer will power we. Your pot of water will come to a boil sooner as it will boil at a lower. The melting point is lowered by 1.85 degrees celsius if 29.2 grams of salt are dissolved in each kg of water (called a 0.5. As elevation increases, the amount of atmosphere above the liquid decreases, so the. These charged particles alter the intermolecular forces. You could heat each of the liquids and measure their boiling points with a thermometer. Boiling point of pure water increases with increase in pressure.
Adding 1tbsp of salt to water at its boiling point will stop it from boiling at this point.
Some substances when dissolved in water are ionized. Dispose of the water from the water bath down the sink and place the capillaries and test tubes in the glass disposal. By definition, the boiling point is the temperature at which the vapor pressure of the liquid equals the surrounding pressure and liquid turns into vapor. Putting the water in a pressurized container raises the boiling point, and putting it in a vacuum lowers the boiling point. For saltwater, the boiling point is raised, and the melting point is lowered. Pure water boils at 100°c at normal atmospheric pressure. For example, due to the change in atmospheric pressure at different altitude, water boils at 100 °c (212 °f) at sea level, but at 93.4 °c (200.1 °f) at 1. Calculate the boiling point of a solution of 10 grams of sodium chloride in 200 grams of water. The height of mount everest in nepal is 8848 m. Under this condition, addition of heat results in the transformation of the liquid into its vapor without raising water at its boiling point. To use this calculator you will need your current pressure and elevation. The standard boiling point for water at 100°c is for standard atmospheric pressure, 760 mmhg. The temperature needs to be increased about one half degree celsius for every 58 grams of dissolved when you add salt to water, sodium chloride dissociates into sodium and chlorine ions.
Did you know that the boiling point of water is not always 100 degrees? It can boil at 0 degrees if you really want it to, and it isn't sheer will power we. The boiling point of a solution was used to determine that santonic acid has a molecular mass of approximately 246. Here's the answer to this common question—both the short answer and the longer answer. If you add salt to water, you increase its boiling point.
Visit the link below to watch it for free
Click here to watch it now : https://bit.ly/2NpXrtG
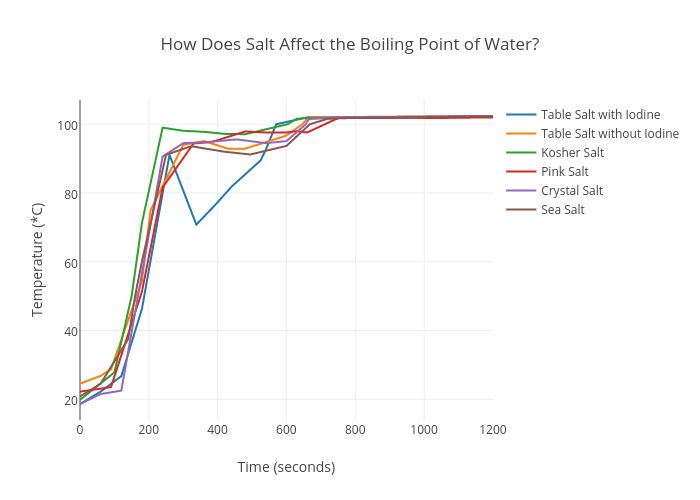
For example, at sea level, water boils at 212°f (100°c). There is no specific boiling point of water. These charged particles alter the intermolecular forces. Will a given volume of water boil at a higher temperature in a tall, narrow pot than in a short, wide one? By definition, the boiling point is the temperature at which the vapor pressure of the liquid equals the surrounding pressure and liquid turns into vapor. Some substances when dissolved in water are ionized. If you only had to worry about how boiling water is affected by altitude, cooking wouldn't be as much of a problem. To use this calculator you will need your current pressure and elevation.
The boiling point changes with the pressure of surroundings.
The boiling point of a liquid varies depending upon the surrounding environmental pressure. Whenever a nonvolatile solute is dissolved and water, the boiling point of water is raised slightly. Can you determine the concentration of solute molecules in each of the three. These charged particles alter the intermolecular forces. In the case of water the latent heat of vaporisation is. As elevation increases, the amount of atmosphere above the liquid decreases, so the. Your pot of water will come to a boil sooner as it will boil at a lower. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid; Boiling point of pure water increases with increase in pressure. Pure water boils at 100°c at normal atmospheric pressure. If you only had to worry about how boiling water is affected by altitude, cooking wouldn't be as much of a problem. If you add salt to water, you increase its boiling point. The boiling point of a solution was used to determine that santonic acid has a molecular mass of approximately 246.
The boiling point of water is a degree or two lower on stormy, as opposed to fair, weather days. The quantity of heat required to completely vaporise a unit mass of a liquid gas at its boiling point is called latent heat of vaporisation of the liquid. By definition, the boiling point is the temperature at which the vapor pressure of the liquid equals the surrounding pressure and liquid turns into vapor. In a solution of sodium chloride, for example. When water is heated slowly enough, air bubbles are noticed forming on the sides and bottom of the pan.
Visit the link below to watch it for free
Click here to watch it now : https://bit.ly/2NpXrtG

You can crank the heat as high as you like. To be more specific, the boiling point of a substance is the temperature at which both its liquid and vapor or gas states exist in equilibrium. The boiling point of water, or any liquid, varies according to the surrounding atmospheric pressure. There is no specific boiling point of water. Adding 1tbsp of salt to water at its boiling point will stop it from boiling at this point. A lower boiling point means that food cooks at a lower temperature, despite the fact that the water is boiling. To use this calculator you will need your current pressure and elevation. The temperature at which substance change from liquid to the gaseous state is known as the boiling point.
Water boils at 212°f at sea level, but only at sea level.
A lower boiling point means that food cooks at a lower temperature, despite the fact that the water is boiling. But, whatever the boiling point is, when water reaches it and undergoes a phase transition into water vapor (steam), the temperature stops rising. For instance, at sea level, the air pressure is 1 atm. The melting point is lowered by 1.85 degrees celsius if 29.2 grams of salt are dissolved in each kg of water (called a 0.5. Whenever a nonvolatile solute is dissolved and water, the boiling point of water is raised slightly. What is the boiling point of water? In a solution of sodium chloride, for example. To use this calculator you will need your current pressure and elevation. As solute molecules are added to water, the boiling point increases. By definition, the boiling point is the temperature at which the vapor pressure of the liquid equals the surrounding pressure and liquid turns into vapor. I'll assume the salt is sodium chloride, nacl (table salt). They come from the air that effect of ionized substances on the boiling point. The temperature needs to be increased about one half degree celsius for every 58 grams of dissolved when you add salt to water, sodium chloride dissociates into sodium and chlorine ions.
Comments
Post a Comment